Crigler-Najjar Syndrome
Information and useful links on Crigler-Najjar syndrome
Definition
Bilirubin is the waste product of the breakdown of hemoglobin occurring during the normal turnover of red blood cells. Bilirubin is not soluble in water and before excretion in the bile must be associated with a substance called glucuronid acid. This process takes place in the liver thanks to the bilirubin-uridine diphosphoglucuronate glucuronosyltransferase (B-UDPGT), also known as UDP-glucuronosyltransferase 1A1 (UGT1A1) enzyme. In Crigler-Najjar patients the enzyme is either inactive (type I) or severely reduced (type II). Therefore, bilirubin cannot be excreted into the bile and remains in the blood. The high plasma level of unconjugated bilirubin leads to jaundice and may lead to bilirubin-induced neurologic damage (kernicterus, bilirubin encephalopathy) due to bilirubin toxicity.
Clinical Manifestation
 the skin and the whites of the eyes become yellow. This is due to excessive plasmatic levels of bilirubin (> 20 mg/dL, corresponding to approximately 342 umol/L, in Crigler-Najjar type I patients). Whether untreated severe jaundice may result in brain damage (kernicterus) with possible permanent effects. As results of brain damage clinical manifestations of kernicterus include hypotonia, lethargy, deafness, oculomotor palsy.
the skin and the whites of the eyes become yellow. This is due to excessive plasmatic levels of bilirubin (> 20 mg/dL, corresponding to approximately 342 umol/L, in Crigler-Najjar type I patients). Whether untreated severe jaundice may result in brain damage (kernicterus) with possible permanent effects. As results of brain damage clinical manifestations of kernicterus include hypotonia, lethargy, deafness, oculomotor palsy. Genetics
Several gene alterations have been discovered in Crigler-Najjar syndrome patients, leading to reduced or absent UGT1A1 activity causing hyperbilirubinemia. Full-length cDNA for human UGT1A1 has been cloned and sequenced.
Crigler-Najjar syndrome is very rare, real incidence is unknown (approx. less than 1 case per 1,000,000 births).
Bilirubin
| Indirect Bilirubin (Unconjugated) | Direct Bilirubin (Conjugated) |
| Also referred as: insoluble, prehepatic, indirect-reacting, albumin-bound bilirubin | Also referred as: posthepatic, direct-reacting, unbound bilirubin |
| Normal plasmatic range: 0.2 - 0.9 mg/dL (see note below) | Normal plasmatic range: 0.1 - 0.2 mg/dL (see note below) |
| Derives mainly from hemoglobin metabolism | It is the product of bilirubin metabolism within the liver |
| Hydrophobic | Water soluble |
| Must be conjugated in the liver before it can be excreted | Is readily excreted into bile, stool, and urine |
| Most bound to albumin in plasma; a small amount free in the plasma | Only a small amount normally found within the plasma |
| Never present in the urine | May be found in the urine when serum levels exceed 3-4 mg/dL (see note below) |
| Can be measured only indirectly, by subtracting the direct bilirubin level from the total bilirubin level | Can be measured directly in plasma |
Total serum bilirubin (referred as total bilirubin) equals the sum of direct (conjugated) and indirect (unconjugated) bilirubin.
Note: Milligrams/deciliter (mg/dL) is the unit of measure commonly used for bilirubin. Micromoles/liter (umol/L) is the designated Systeme International (SI) unit of measure recommended for reporting clinical laboratory results .
- 1 mg/dL of bilirubin is 17.1 umol/L. (see bilirubin unit converter calculator)
Due to the absence of the activity of the UGT1A1 enzyme (which is the liver enzyme involved in the process of transformation of unconjugated bilirubin to conjugated bilirubin) Crigler-Najjar type I patients have high levels (above 20 mg/dL) of unconjugated bilirubin and very low levels or absence of conjugated bilirubin.
Treatment
To improve effectiveness of phototherapy it is best to:

- Change lamps after about 1,000-1,500 hours of use (approximately every four to six months)
- Keep the light source close to the body (about 15-20 centimeters, 6-8 inches)
- Maximize skin exposure to light
- Use solid white sheets
- Place reflective surfaces (mirrors and emergency blankets) around the bed (you might find more information in the Phototherapy page)
Hepatocyte transplantation (transplantation of hepatic cells, i.e. hepatocytes, rather than whole liver) has been performed on a limited number of patients as an experimental therapeutic procedure (you might find some reports in the news page). Major limitations hampering a more generalized clinical application of this option include the transient benefit in the correction of the disorder, the need of suitable sources for cell isolation and technical problems such as criopreservation of isolated cells.
Other treatments aiming at reducing bilirubin entero-hepatic circulation have been proposed including oral administration of calcium phosphate, cholestyramine and agar. During 2007 results of a clinical trial using oral administration of Orlistat have been published. These data indicated an increased fecal excretion of fat and unconjugated bilirubin with concomitant decreased levels of plasmatic unconjugated bilirubin.
Gene therapy is considered a promising experimental option. During 2017-2018 several clinical trials have been proposed (you might find more information in the Gene Therapy Clinical Trials page below)
Phototherapy
 The mechanism of bilirubin excretion during phototherapy. BR: bilirubin. PBR: photobilirubin (from MacDonagh et al, Science 1980).
The mechanism of bilirubin excretion during phototherapy. BR: bilirubin. PBR: photobilirubin (from MacDonagh et al, Science 1980).
Current treatment for Crigler-Najjar patients is based upon phototherapy (emission range: 400-525 nm, peak emission: 450-460 nm). Patients undergo to phototherapy sessions (about 10 hours/day). Light energy is absorbed by bilirubin as it circulates in skin capillaries, resulting in conversion of insoluble bilirubin to photoisomers (referred also as photobilirubin and lumirubin) which are water-soluble and readily excreted.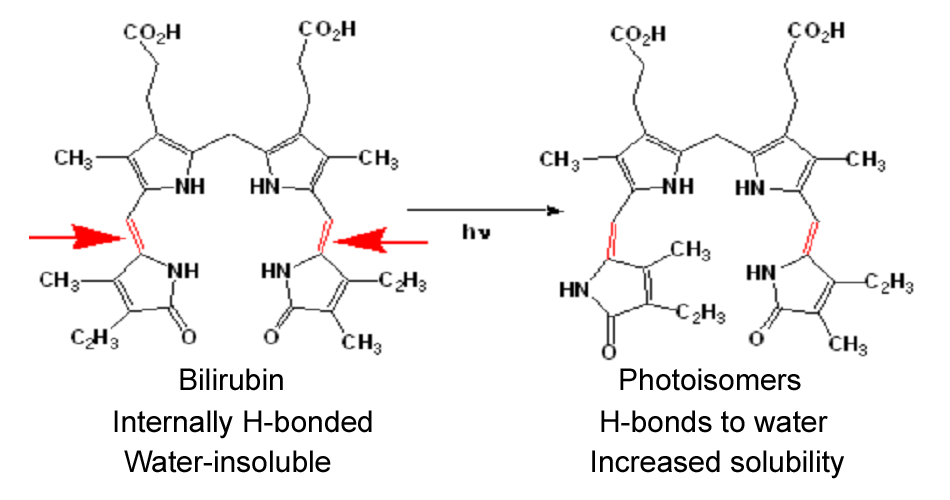 Bilirubin photoisomerizationDifferent parameters are to be considered about phototherapy:
Bilirubin photoisomerizationDifferent parameters are to be considered about phototherapy:
LIGHT SOURCE:
Fluorescent lamps
Philips Special Blue lamps F20T12/BB or F40/BB (which are different from "regular" blue lamps labeled F20T12/B) or Philips TL 52 lamps are generally used as light source.
Factors affecting the effectiveness of phototherapy:
- Lamps life (change lamps after about 1,000 operating hours)
- Distance from the light source (ideally 4-8 inches, 10-20 cm). The more the distance, the less is the light energy hitting the body surface.
- Skin exposure to light (not less than 40% of body surface has to be exposed to light)
- Surface of the skin exposed/body weight ratio
- Pigmentation
- Duration of the exposure (the effectiveness of phototherapy is to some extent proportional to the phototherapy time)
- Use of reflective surfaces around the bed and white sheets to reduce absorption
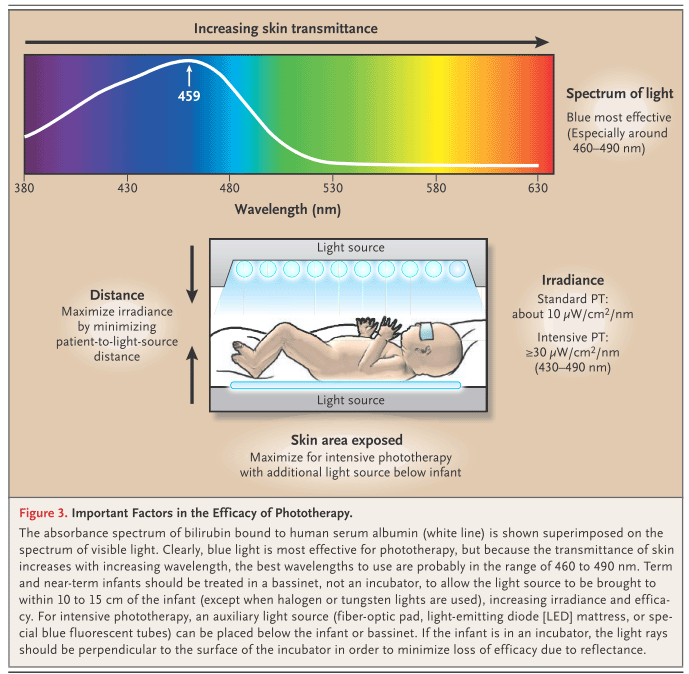 From Maisels and McDonagh, N. Engl. J. Med. 2008; 358:920-8.Light Emitting Diodes - LEDs
From Maisels and McDonagh, N. Engl. J. Med. 2008; 358:920-8.Light Emitting Diodes - LEDs
In the past years, technology of phototherapy devices moved from fluorescent lamps to LED as source of light.
The efficacy of LED lights in reducing total serum bilirubin levels is comparable to that of conventional light sources (fluorescent or halogen lamps).
Moreover, compared to conventional fluorescent lamps, LED have the following advantages:
- Emission peak precisely around the bilirubin absorption peak (reducing problems arising from infra-red and ultra-violet light exposure)
- Prolonged lifetime (up 20,000-50,000 hours)
- Energy saving (more cost-effective)
- Reliability (same emission profile throughout LED lifetime)
- Easier to build phototherapy devices (even including LED in blankets)
- Reduced heat emission (therefore can be applied closer to the body, increasing efficacy)
- Exhausted LED are more easily disposable
HOW TO BUILD A PHOTOTHERAPY UNIT:
Another problem to face is how to build a phototherapy unit for adult patients with limited costs. Here we show how some ingenious dads have solved the problem of home phototherapy for their children
(Click on the picture to see a larger view)
 Made by Alex (e-mail: alexcar@bigpond.com)
Made by Alex (e-mail: alexcar@bigpond.com) Made by Alex (e-mail: alexcar@bigpond.com)
Made by Alex (e-mail: alexcar@bigpond.com)
 Made by Gaia's dad (e-mail: info@ciami.it )
Made by Gaia's dad (e-mail: info@ciami.it )
 Made by Graham (e-mail: Graham)
Made by Graham (e-mail: Graham)
 Made by The University of Twente, The Netherlands
Made by The University of Twente, The Netherlands
 Ledwrap made by Philomeen Engels
Ledwrap made by Philomeen Engels
 LED blanket by Smart Texiles
LED blanket by Smart Texiles
 Home phototherapy system using Royal Blue power LEDs made by Charles Murphy (e-mail: Charles Murphy)
Home phototherapy system using Royal Blue power LEDs made by Charles Murphy (e-mail: Charles Murphy)
 Bililed Blue Night phototherapy unit using LEDs
Bililed Blue Night phototherapy unit using LEDs
 Led lamp by Dutch Medical Technology
Led lamp by Dutch Medical Technology
 Phototherapy crib mattress from Archives of Disease in Childhood
Phototherapy crib mattress from Archives of Disease in Childhood
Daytime Phototherapy Unit
How to build a sit up unit.
Some companies have developed experimental sunbeds designed for adult Crigler-Najjar patients. Among those
- Bililed, NL. LED phototherapy lamps for Crigler-Najjar syndrome
- GoldenLite, UK
- Medestime, France
- ST. Bachmann, Germany
- Standford University, USA
- Experimental portable phototherapy device
Other interesting sites:
- For comprehensive answers about phototherapy for CN patients please refer to the following site: www.criglernajjar.info
- Phototherapy page of the French Crigler Najjar Association (in French).
- Katelyn's light.
- Pictures of phototherapy units (from the Italian Crigler-Najjar Association).
Gene Therapy Clinical Trials
Gene therapy to treat Crigler Najjar syndrome. aims to introduce a correct copy of the UGT1A1 gene into the patient's liver cells. This procedure can be accomplished by the administration of specific "vectors" which carry the genetic material to be delivered. Preclinical studies performed on the animal model of the disease (the Gunn rat) provided evidence that gene therapy may promote the correction of the disease.
Several gene therapy clinical trials are being considered as a therapeutic option for the treatment of Crigler-Najjar patients:
Title: A phase I/II, open label, escalating dose study to evaluate safety and efficacy of an intravenous injection of GNT0003 (Adeno-associated Viral Vector expressing the UGT1A1 transgene) in patients with severe Crigler-Najjar syndrome requiring phototherapy.
Abbreviated trial name: CareCN - CureCN
Purpose: To assess safety, tolerability and efficacy of a single intravenous administration of Adeno-associated Viral Vector expressing the UGT1A1 transgene in patients with severe Crigler-Najjar syndrome requiring phototherapy.
Sponsor: Genethon - Developer: Genethon and Spark Therapeutics
Sponsor:This project has received funds from the European 's Union Horizon 2020 research and innovation programme under the grant agreement number 755225 Cure CN Horizon 2020 Project
EU Clinical Trial Register number: 2017-000506-37
EU Clinical Trial Link: https://www.clinicaltrialsregister.eu/ctr-search/trial/2017-000506-37/NL
ClinicalTrials.gov Identifier: NCT03466463
ClinicalTrial.gov Link: https://clinicaltrials.gov/ct2/show/NCT03466463?cond=Crigler-Najjar+Syndrome&rank=1
December 2018: The first patient affected by Crigler-Najjar syndrome administered with gene therapy vector in CURE CN trial sponsored by Genethon. A second patient has been treated. No significant side effects were reported, but the reduction of hyperbilirubinemia in both treated patients was transient.
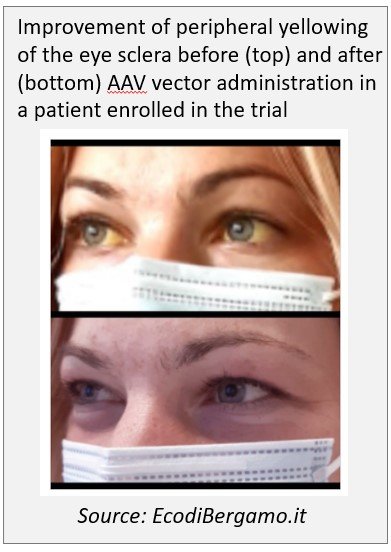
November 2020: A patient affected by Crigler-Najjar syndrome type I underwent gene therapy treatment with an higher dose of viral vector at the Papa Giovanni XXIII Hospital in Bergamo (Italy) by the Prof. Lorenzo D'Antiga's team. March 2021: A second patient has been treated on March 2021, a third on June 2021.
June 2021: Exciting results achieved so far in AAV-mediated gene therapy trial for Crigler Najjar syndrome by Prof. D'Antiga at the Congress of the European Association for the Study of the Liver. In particular, the treatment was well tolerated in the patients treated so far. The administration of the vector at the highest dose resulted in a significant reduction in unconjugated bilirubin levels, allowing the first treated patient to stop phototherapy. The study is still ongoing
Last Update March 2023: Genethon Given PRIME Status by EMA for Gene Therapy To Treat Crigler-Najjar Syndrome
August 2023: Published on New England Journal of Medicine the results of a phase 1-2 study evaluating the safety and efficacy of a single intravenous infusion of an adeno-associated virus serotype 8 vector encoding UGT1A1 in patients with the Crigler-Najjar syndrome that was being treated with phototherapy.

Link to a press release from Genetic Engineering and Biotechnology News
Title: Clinical Assessment Study in Crigler-Najjar Syndrome
Abbreviated trial name: LUSTRO
ClinicalTrials.gov Identifier: NCT03078881
Purpose: Pre-Phase 1 prospective, non-interventional clinical assessment study to evaluate Crigler-Najjar syndrome subjects requiring daily phototherapy, aged 1 year and older.
Sponsor: Audentes Therapeutics (Astallas)
ClinicalTrials.gov Identifier: NCT03078881
ClinicalTrials.gov Link: https://clinicaltrials.gov/ct2/show/NCT03078881
Title: Gene Transfer Clinical Study in Crigler-Najjar Syndrome (VALENS)
Abbreviated trial name: VALENS
Purpose: Phase 1/2, multinational, open-label, ascending-dose, delayed-treatment concurrent control clinical study to evaluate the safety and preliminary efficacy of adeno-associated viral vector expressing the UGT1A1 transgene in subjects with Crigler-Najjar aged ≥1 year.
Sponsor: Audentes Therapeutics
ClinicalTrials.gov Identifier: NCT03223194
ClinicalTrials.gov Link: https://clinicaltrials.gov/ct2/show/NCT03223194
LAST UPDATE: February 2018: First patient affected by Crigler-Najjar syndrome administered with gene therapy vector in VALENS clinical trial sponsored by Audentes. Later the trial was suspended.
Crigler Najjar disease type II
Crigler-Najjar syndrome has been classified into two types according to the degree of hyperbilirubinaemia and to the response to phenobarbital administration.
The more severe Crigler-Najjar type I (Online Mendelian Inheritance in Man #218800 ) is characterized by severe chronic non-haemolytic unconjugated hyperbilirubinaemia with high levels of serum bilirubin (between 20-50 mg/dL) due to the absence of bilirubin B-UDPGT (UGT1A1) activity.
In the milder Crigler-Najjar type II (known also as Arias syndrome) (Online Mendelian Inheritance in Man #606785 ), bilirubin UGT1A1 activity is only decreased and a consistently significant reduction of hyperbilirubinemia is obtained with phenobarbital treatment, which does not occur in Crigler-Najjar type I. The response to phenobarbital may represent induction on the residual UGT1A1 activity present in type II patients.
Clinically, Crigler-Najjar type I and type II syndrome are discriminated on the basis of the following clinical criteria:
- Type I patients do not respond to phenobarbital treatment and only traces of bilirubin glucuronides can be found in their bile.
- In type II, phenobarbital treatment (3-5 mg/kg/die, single administration) lowers serum bilirubin levels by more than 30%.
- In type II bilirubin glucuronides are present in bile.
- Analysis of liver tissue reveals residual activity of UGT1A1 activity in type II and absent activity in type I.
| Crigler Najjar type I | Crigler Najjar type II | |
| Serum bilirubin concentration | 20-50 mg/dL | < 20 mg/dL |
| Hepatic UGT1A1 activity | Absent | Markedly reduced |
| Effect of phenobarbital on serum bilirubin concentration | None | Reduction |
| Bile | Usually pale: contains small amounts of unconjugated bilirubin | Increased proportion of bilirubin monoglucoronide |
| Prognosis | Kernicterus | Usually benign |
| Mode of inheritance | Autosomal recessive | Most likely autosomal recessive |
Several alteration in the UGT1A1 gene have been described both in Crigler-Najjar type I and Crigler-Najjar type II patients (see The Human Gene Mutation Database). As a general rule mutations in Crigler-Najjar type I patients disrupt completely UGT1A1 activity, while mutations discovered in Crigler-Najjar type II patients have a milder effect on the protein activity even if there is considerable variability in type II, making it difficult to classify some cases.
Crigler-Najjar type I is inherited as an autosomal recessive trait. Also type II is believed to be autosomal recessive even if the pattern of inheritance is not certain. For Crigler-Najjar type II, in fact, also an autosomal dominant transmission model has been proposed.
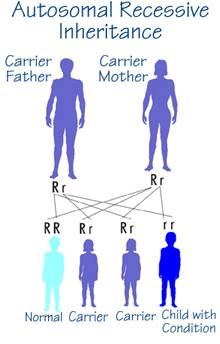
In an autosomal recessive inheritance two copies of an altered gene located on one of the autosomes (that is, not the X or Y chromosomes) must be present for an individual to be affected with the trait or condition determined by that gene.
- An affected individual (homozygote) has two parents who are unaffected but each parent carries the altered gene (heterozygote).
- The risk of two heterozygotes, or carriers, having an affected child is 25%, 1 in 4, for each child that they have; similarly, there is a 3 in 4 chance (75%) that each child will not be affected.
- Males and females are at equal risk for being affected.
- Two affected individuals (homozygotes) usually produce children all of whom are affected as well.
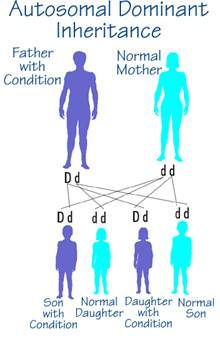
Autosomal dominant inheritance is marked by the primary feature that one copy of an allele is sufficient for expression of a trait; the gene located on one of the 22 autosomes (that is, not the X or Y sex chromosome) is expressed in the heterozygous state.
- Each affected person has at least one affected parent. Exceptions may occur for one of three reasons:
- The affected person is the result of a new mutation.
- The parent transmitting the gene did not show the trait, even though he or she carried the allele; this is known as incomplete penetrance, that is, the inconsistent phenotypic expression of a gene even though the gene is present.
- The parent of the affected individual expressed the gene but in ways that were not readily recognized; this is known as variable expressivity.
Other characteristics of autosomal dominance:
- An affected person has a 50% chance of passing the trait to a child.
- Males and females are equally likely to be affected.
- Two affected people can have an unaffected child.
Related resources:
- Crigler Najjar Type 2 - from the Journal of Formosan Medical Association.
- Crigler Najjar Type II - from the Indian Journal of Pediatrics.
- Molecular pathology of Crigler-Najjar type I and II and Gilbert's syndromes - from Haematologica).
- Crigler-Najjar Syndrome Type 2 in a Young Adult - from J Medicine).
Crigler-Najjar syndrome and pregnancy
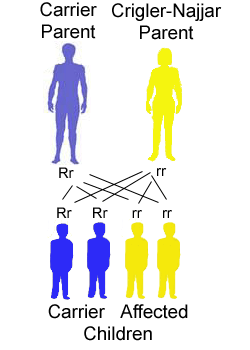
Mutated gene frequency for autosomal recessive disorders is estimated by calculating the square root of the disease frequency. Considering that Crigler-Najjar disease occurs in 1 in 1,000000, the altered gene frequency is 1:1000. Therefore, the frequency of unaffected persons carrying the mutated UGT1A1 gene (defined as carrier or heterozygote) is 1:500, since we have two copies of each autosomal gene. Carriers for a mutation in the Crigler-Najjar syndrome gene have about half the UGT1A1 enzyme activity of a normal adult. Despite that, since along with the mutated copy they have a copy of the "correct" gene, they do not have significant consequences in the bilirubin levels.
With genetic testing (see below) it is possible to determine whether a person has a mutation in the UGT1A1 gene.
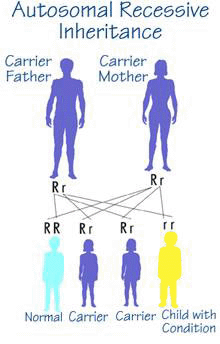
A Crigler-Najjar patient (homozygote) has two parents who are unaffected but each parent carries an altered (mutated) copy of the UGT1A1 gene along with a "correct" copy of the gene (heterozygote).
The risk for two heterozygotes (carriers) to have an affected child is 25%, 1 in 4, for each child that they have. In this case the child takes the mutated copy of the gene from each parent. Similarly, there is a 3 in 4 chance (75%) that each child will not be affected by the disease.
Males and females are at equal risk for being affected by Crigler-Najjar syndrome.
 Before the use of phototherapy as therapeutic option, Crigler-Najjar syndrome type I was fatal during childhood. Nowadays, patients compliant with this kind of treatment can reach adulthood.
Before the use of phototherapy as therapeutic option, Crigler-Najjar syndrome type I was fatal during childhood. Nowadays, patients compliant with this kind of treatment can reach adulthood.Whether followed by constant medical monitoring Crigler-Najjar patients may successfully give birth to healthy babies.
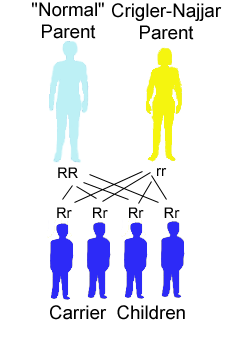
The risk for a Crigler-Najjar patient (homozygote) having a baby with a unaffected (not carrier) person to have a child affected with the disease is 0%. All children will be carrier (heterozygote) with a copy of the mutated gene and a copy of the correct gene.
 As said above, in general population (i.e. in absence of a family history of Crigler-Najjar syndrome) the chance for an unaffected person to have a mutation in the UGT1A1 gene (in other words to be a carrier of the disease) is 1:500.
As said above, in general population (i.e. in absence of a family history of Crigler-Najjar syndrome) the chance for an unaffected person to have a mutation in the UGT1A1 gene (in other words to be a carrier of the disease) is 1:500.The risk for a Crigler-Najjar patient (homozygote) having a baby with an unaffected carrier (heterozygote) person to have a child affected with the disease is 50%, for each child they have. There is a remaining 50% chance that the children will be unaffected carriers.
 It has been reported that hyperbilirubinemic female Gunn rats (the animal model of Crigler-Najjar syndrome) have reduced fertility (Davis D.R., Yeary R.A. Impaired fertility in the jaundiced female Gunn rat. Lab Anim Sci. 1979;29:739-43). No data are available on fertility of Crigler-Najjar patients.
It has been reported that hyperbilirubinemic female Gunn rats (the animal model of Crigler-Najjar syndrome) have reduced fertility (Davis D.R., Yeary R.A. Impaired fertility in the jaundiced female Gunn rat. Lab Anim Sci. 1979;29:739-43). No data are available on fertility of Crigler-Najjar patients.Here we give links to several clinical reports dealing with patients affected by Crigler-Najjar syndrome during pregnancy.
Case Reports:
- Creeper et al. Crigler-Najjar type II in pregnancy: A case report. Obstet Med 2023; 16(3):184-186.
- Chaubal et al. Management of pregnancy in Crigler Najjar syndrome type 2. World J Hepatol 2016; 8(11): 530-532
- Chaudhary et al. A rare case of Crigler-Najjar syndrome type II with pregnancy. Int J of Reprod Contracept Obstet Gynecol 2014; 3(1): 261-262
- Wilson et al. Recommendations for pregnancies in patients with Crigler-Najjar Syndrome. JIMD Rep. 2013; 7: 59–62.
- Passuello et al. Pregnancy outcome in maternal Crigler-Najjar Syndrome Type II: a case report and systematic review of the literature. Fetal Diagnosis and Therapy, 2009;26:121–126
- Arora N, Choudhary S. Pregnancy with Crigler-Najjar syndrome type II. Journal of Obstetrics and Gynaecology, 2009; 29: 242-244.
- Hannam S, Moriaty P, O’Reilly H, Craig JS, Heneghan MA, Baker A and Dhawan A. Normal neurological outcome in two infants treated with exchange transfusions born to mothers with Crigler-Najjar Type 1 disorder. European Journal of Pediatrics. 2008; 16: 427-429.
- Gajdos V, Petit F, Trioche P, Mollet-Boudjemline A, Chauveaud A, Myara A, Trivin F, Francoual J, Labrune P. Successful pregnancy in a Crigler-Najjar type I patient treated by phototherapy and semimonthly albumin infusions. Gastroenterology. 2006; 131: 921-924.
- Pinkee S, Renu A, Bharati M. Crigler Najjar syndrome with pregnancy. J. Obstet. Gynaecol. India. 2005; 55: 270-271.
- Holstein A, Plaschke A, Lohse P, Egberts EH. Successful photo-and phenobarbital therapy during pregnancy in a woman with Crigler-Najjar syndrome type II. Scand J Gastroenterol. 2005; 40: 1124-1126.
- Ito T, Katagiri C, Ikeno S, Takahashi H, Nagata N, Terakawa N. Phenobarbital following phototherapy for Crigler-Najjar syndrome type II with good fetal outcome: a case report. J Obstet Gynaecol Res. 2001; 27: 33-35.
- Smith JF, Jr., Baker JM. Crigler-Najjar disease in pregnancy. Obstet Gynecol. 1994; 84: 670-672.
- Taylor WG, Walkinshaw SA, Farquharson RG, Fisken RA, Gilmore IT. Pregnancy in Crigler-Najjar syndrome. Case report. Br J Obstet Gynaecol. 1991; 98: 1290-1291.
Seek genetic counseling if you are planning a pregnancy and you are affected or have a family history of Crigler-Najjar syndrome.
MOLECULAR DIAGNOSIS FOR CRIGLER-NAJJAR SYNDROME:
Genetic testing, including prenatal diagnosis and preimplantation genetic diagnosis , for Crigler-Najjar syndrome type I and type II patients and relatives are possible.
To find laboratories specialized in molecular diagnosis refer to the following websites:
Drugs which may interfere with bilirubin levels
Some drugs may interfere with the bilirubin level. Plasmatic bilirubin is generally associated with albumin, thanks to specific binding sites. Some drugs may compete for these binding sites, therefore indirectly increasing the level of unbound bilirubin in the blood. As a conseguence the bilirubin/albumin molar ratio may change with an increased risk of development of kernicterus (see here for more on bili/alb molar ratio). In facts, the complex bilirubin-albumin is not able to cross the hematoencephalic barrier; on the contrary, unbound bilirubin may cross the hematoencephalic barrier. In other words, if the serum level of bilirubin exceeds the binding capacity of albumin, the lipid-soluble free bilirubin crosses the blood-brain barrier, causing a neurologic condition referred as kernicterus.
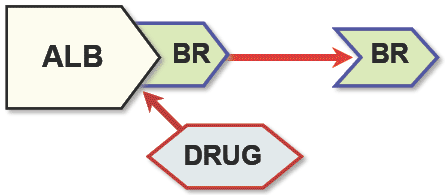 Schematic view of the mode of action of bilirubin-albumin displacing drugs (from Strauss et al.)
Schematic view of the mode of action of bilirubin-albumin displacing drugs (from Strauss et al.)The following table (from http://micromedex.hcn.net.au/mdx-29191/ddl45.htm) is a list of drugs which may interfere with the bilirubin level. Consider it as a reminder to always ask you physician or pharmacist before assuming any drug to check if it is right for you. For more infos on potential bilirubin-albumin displacing drugs see also appendix in Strauss et al. 2006
| Acetaminophen | Erythromycin (estolate, ethylsuccinate) | Penicillamine |
| Anabolic steroids | Ethambutol | Phenazopyridine |
| Azathioprine | Etretinate | Phenothiazines: Chlorpromazine Fluphenazine Perphenazine Prochlorperazine Thioridazine. |
| Benzodiazepines: Chlordiazepoxide, Diazepam. | Fusidic acid | Propoxyphene |
| Carbamazepine | Halothane | Pyrazinamide |
| Chloral hydrate | Idoxuridine | Rifampin |
| Chlorpropamide | Immune globulin | Spectinomycin |
| Cyproheptadine | Methyldopa | Sulfonamides: Cotrimoxazole |
| Dantrolene | Methylene blue (neonates) | Sulfonylureas: Tolbutamide |
| Dapsone | Nitrofurantoin | Tetracyclines: Demeclocycline, Minocycline, Oxytetracycline, Ticlopidine. |
| Dinoprostone(neonatal) | Nonsteroidal anti- inflammatory agents: Benoxaprofen, Diclofenac, Diflunisal, Ibuprofen, Phenylbutazone. | Ticlopidine |
| Disopyramide | Oral contraceptives | Tricyclic antidepressants:: Amitriptyline, Desipramine, Imipramine |
| Enflurane | Paraaminosalicylic acid | Valproic acid |
From: http://micromedex.hcn.net.au/mdx-29191/ddl45.htm
Links from: http://www.nlm.nih.gov/medlineplus/druginformation.html
Animal models of the Disease
 The Gunn rat
The Gunn ratAn a
 Gunn rat serum sampleccurate model of the disease, the Gunn rat, exists; the UGT1A1 gene in this strain carries a premature stop, leading to a very low UGT1A1 activity.
Gunn rat serum sampleccurate model of the disease, the Gunn rat, exists; the UGT1A1 gene in this strain carries a premature stop, leading to a very low UGT1A1 activity.Charles Kenneth Gunn first discovered mutant rats in 1934 (published in 1938) in a Wistar rat colony maintained at the Connaught Laboratory in Toronto, Canada. Rats were jaundiced and the defect was transmitted as an autosomal recessive characteristic. It took several decades to fully elucidate at the molecular level the deficiency in the Gunn rats. The human counterpart of the disease was described by John Fielding Crigler and Victor Assad Najjar in 1952.Various Gunn rat stocks were derived from those animals and have genetic contributions of different strains.


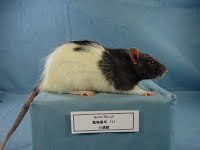
Gunn rats pups and adult animals (homozogous rats designed by jj) have a yellowish discoloration of the skin (jaundice).Gunn rats are commercially available from Harlan, Indianapolis, Indiana. Gunn rats are also available from Japan SLC Inc.. This animal model has been estremely valuable for the development of experimental treatments for the disease.
Recently, a mouse knock-out model (Ugt1-/- mice), in which the UGT1 locus has been disrupted has been created. In Ugt1-/- mice unconjugated levels of bilirubin are very high. These animals generally die within 2 weeks after birth. Ugt1-/- pups are identified by their yellowish skin color as early as 12 hours after birth
 from Nguyen et al., 2008
from Nguyen et al., 2008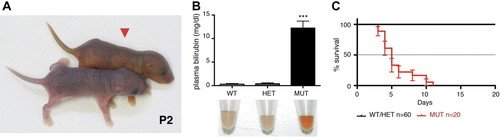 from Bortolussi et al., 2012
from Bortolussi et al., 2012 Patients Associations
THE CRIGLER NAJJAR ASSOCIATION (USA) 
CNA / King’s Way Foundationc/o Cory Mauck3134 Bayberry Street Witchita, Kansas 67226
e-mail: mauckc@msn.com
THE CRIGLER NAJJAR ASSOCIATION (ITALY)

CI AMI Crigler-Najjar Italia Associazione Malati Iperbilirubinemici
Via Ivo Peli, 21
40033 Casalecchio di Reno (Bologna) Italy.
e-mail: info@ciami.it
Web Page: http://www.ciami.it
Facebook: https://www.facebook.com/groups/81323304194/permalink/10158130173589195/
Twitter: @assoCN_ita
Youtube channel: https://www.youtube.com/channel/UC5-zEfpZI8QXSMWsh5L5u-A
THE CRIGLER NAJJAR ASSOCIATION (FRANCE) Associacion Française de Crigler-Najjar
8 rue Henri 92140 CLAMART (FRANCE)
e-mail: crigler-najjar@9online.fr
Web Page: http://www.crigler-najjar.fr/
Facebook: https://www.facebook.com/AssociationFrancaiseCriglerNajjar/
THE CRIGLER NAJJAR ASSOCIATION (THE NETHERLANDS)

HET NAJJAR FONDS Het Najjar Fonds Secretariaat
Oranjeweg 34 1241 XS Kortenhoef (NL)
tel: 035 - 6564121
e-mail: secretariaat@najjar.nl
Web Page: http://www.najjar.nl/
AESCN - Asociación Española de Síndrome de Crigler Najjar
C./ Cañada Rosal, 10 B 33519 - Asturias (Asturias)
Teléfono: 985 724 832
e-mail: laplazacorreo@yahoo.es
Web Page: http://www.geocities.ws/sindromecriglernajjar/
THE CRIGLER-NAJJAR SYNDROME GROUP
List of parents, patients and healthcare providers, sharing common experiences about Crigler-Najjar syndrome type I and II.
GO TO THE CRIGLER-NAJJAR GROUP

GO TO THE CRIGLER-NAJJAR GROUP FOR KIDS

CRIGLER-NAJJAR on FACEBOOK
Crigler Najjar Syndrome - Pakistan: https://www.facebook.com/CNSPAKISTANAWARENESS/
Martin Crigler Najjar Chile: https://www.facebook.com/martincriglernajjar.chile
Crigler Najjar EU: @CriglerNajjarUE
Crigler-Najjar: https://it-it.facebook.com/pages/category/Local-Business/sindrome-di-crigler-najjar-128554117164845/
on Twitter
 Follow @crigler_najjar
Follow @crigler_najjarFollow @assoCN_ita
Follow: @friends_asso
THE CRIGLER-NAJJAR WORLD REGISTRY
A world registry of Crigler-Najjar patients has been established.
THE CRIGLER-NAJJAR SYNDROME PHOTO-ALBUM
Visit our photo-album


